Table of Contents
ToggleIntroduction
Nitrogen is one of the most important nutrients for sustaining life. It is a fundamental building block of proteins, nucleic acids (DNA and RNA), and chlorophyll, which makes it essential for both growth and metabolism. Despite nitrogen gas (N₂) making up nearly 78% of the Earth’s atmosphere, most living organisms cannot directly use this inert form. Instead, they rely on reactive nitrogen compounds such as ammonium (NH₄⁺), nitrate (NO₃⁻), or organic nitrogen compounds (amino acids, nucleotides).
The nitrogen cycle bridges this gap by transforming nitrogen between its various chemical forms. Central to this process is nitrogen fixation, where atmospheric N₂ is converted into ammonia (NH₃), a form accessible to plants. Microorganisms such as bacteria, archaea, and fungi play a key role in different nitrogen transformations, including ammonification, nitrification, and denitrification.
Since the mid-20th century, human activities such as industrial fertilizer production (Haber-Bosch process), fossil fuel combustion, and intensive agriculture have significantly altered the nitrogen cycle. By 2030, it is expected that human-fixed nitrogen will exceed natural microbial fixation, creating both opportunities and challenges for global ecosystems.
This article explores nitrogen fixation, its processes, and ecological significance, while highlighting its role in sustainable agriculture, soil health, and ecosystem balance.
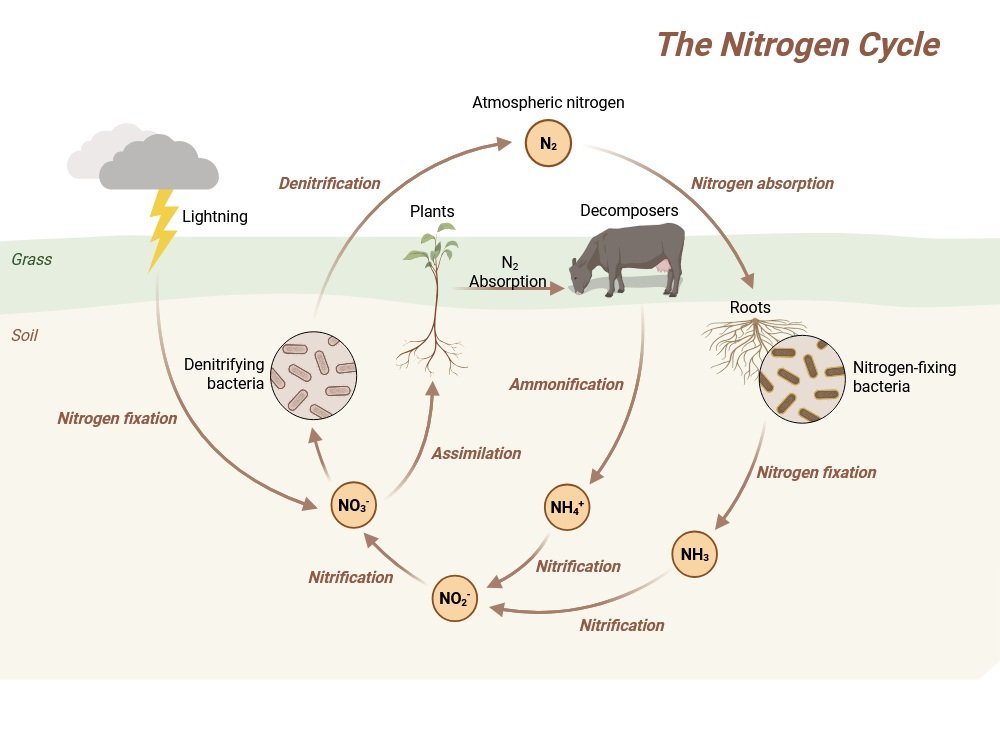
The Process of Nitrogen Fixation
Nitrogen fixation refers to the conversion of atmospheric nitrogen gas (N₂) into ammonia (NH₃) or related compounds. This occurs through biological fixation (microorganisms), industrial fixation, and abiotic natural processes (lightning). However, biological nitrogen fixation remains the most important contributor to ecosystem nitrogen supply.
The nitrogen cycle involves several interrelated processes:
- Ammonification
- Nitrification
- Denitrification
1. Ammonification
- Organic nitrogen is converted into ammonia or ammonium through the process of ammonification.
- It starts with dead organisms, plant waste, animal waste, and other organic material that contains proteins and nucleic acids.
- Extracellular enzymes such as proteases and nucleases degrade these big molecules into smaller molecules such as amino acids and nucleotides.
NH₃ + H⁺ ⇌ NH₄⁺
- Amino acids are stripped of their amino groups by microbial deaminases, which produce α-keto acids as by-products and release ammonia (NH₃).
- The discharged NH₃ is quickly transformed into ammonium (NH₄⁺) in soil and aquatic habitats, where the majority of NH₄⁺ is found under neutral to acidic pH.
- Nitrogen-fixing bacteria may oxidize the produced ammonium, plants and microorganisms can absorb it directly, or it can be absorbed onto soil particles via cation exchange.
- Heterotrophic bacteria and fungi, such as Bacillus, Pseudomonas, and a number of fungal decomposers, carry out the majority of ammonification.
- This process occurs wherever organic nitrogen is broken down, including in compost, sediments, and soils.
2. Nitrification
- Ammonium (NH₄⁺) is biologically oxidized to nitrate (NO₃⁻) in a two-step aerobic process called nitrification.
- The first step involves the conversion of ammonia to nitrite (NO₂) by ammonia-oxidizing bacteria (AOB) like Nitrosomonas and Nitrosospira, as well as ammonia-oxidizing archaea (AOA).
- The enzymes ammonia monooxygenase (AMO) and hydroxylamine oxidoreductase (HAO) catalyze this conversion by oxidizing ammonia to hydroxylamine and then to nitrite.
- The enzyme nitrite oxidoreductase (NXR) is used by nitrite-oxidizing bacteria (NOB), such as Nitrobacter and Nitrospira, in the second step to oxidize nitrite to nitrate.
- The net effect is the formation of acidity in soil as a result of the reaction:
NH₄⁺ + 2 O₂ → NO₃⁻ + H₂O + 2 H⁺.
- Nitrate may be absorbed by plants because it is extremely soluble and mobile, but it is also prone to leaching into groundwater.
- Well-aerated soils and aquatic environments where oxygen is present are where nitrification takes place for the most part.
- It is essential for managing nitrogen availability, soil fertility, and ecological equilibrium.
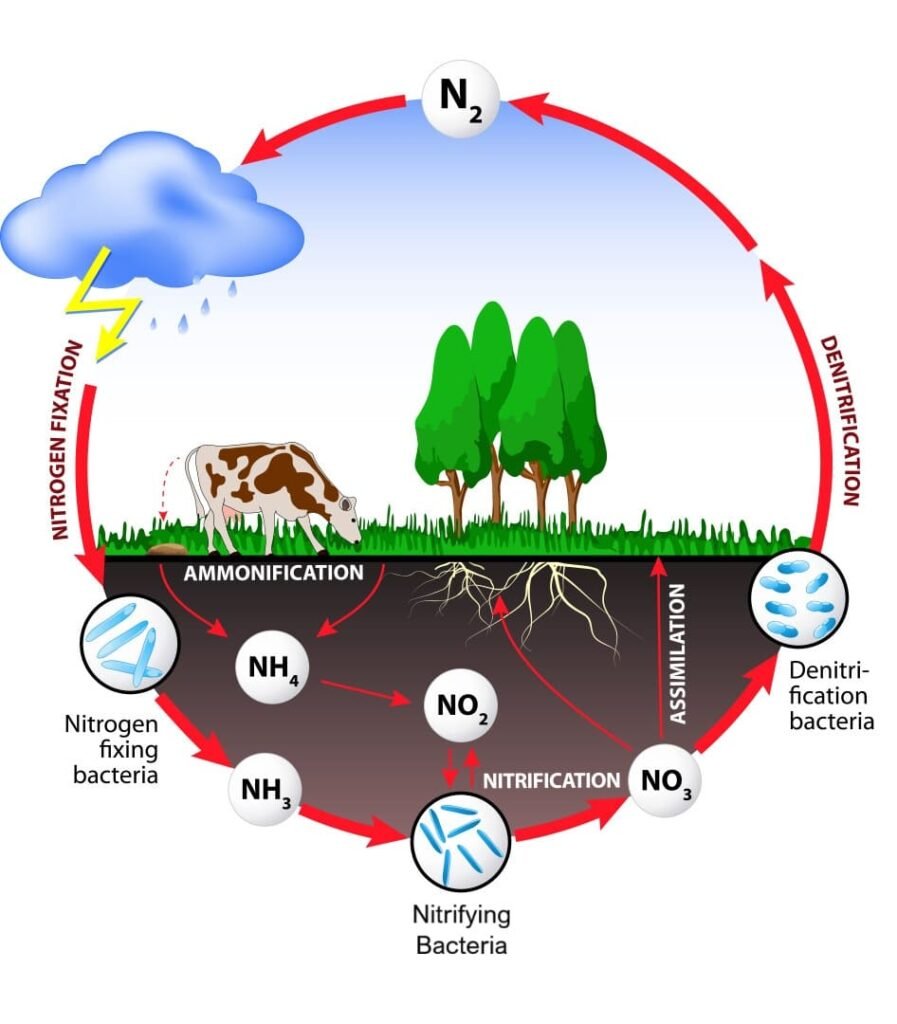
3. Denitrification
- The nitrogen cycle is completed by denitrification, which involves the microbial conversion of nitrate (NO₃⁻) to dinitrogen gas (N₂), thereby returning nitrogen to the atmosphere.
- When microorganisms utilize nitrate instead of oxygen as the terminal electron acceptor, it happens in anaerobic or oxygen-limited environments.
- The reduction sequence is:
Nitrate (NO₃⁻) → nitrite (NO₂⁻) → nitric oxide (NO) → nitrous oxide (N₂O) → dinitrogen gas (N₂)
- The enzymes nitrate reductase (Nar/Nap), nitrite reductase (Nir), nitric oxide reductase (Nor), and nitrous oxide reductase (Nos) catalyze these reactions.
- Facultative anaerobic bacteria like Pseudomonas and Paracoccus carry out denitrification.
- Wastewater treatment facilities, wetlands, sediments, and waterlogged soils are among the places where it may be found.
- The accumulation of too much nitrate in ecosystems is prevented by denitrification.
- Nitrous oxide (N₂O), a strong greenhouse gas and ozone-depleting chemical, can be released by incomplete denitrification.
- The procedure is ecologically essential and has environmental consequences.
Importance of Nitrogen Fixation in the Ecosystem
Nitrogen fixation has profound ecological and agricultural importance:
1. Conversion of Inert Nitrogen to Bioavailable Forms
- Atmospheric nitrogen (N₂) is biologically unavailable.
- Nitrogen fixation provides ammonia (NH₃) and ammonium (NH₄⁺), which can be directly absorbed by plants.
2. Increases Soil Fertility
- Nitrogen-fixing plants such as legumes (peas, clovers, alfalfa, vetches) enhance soil fertility naturally.
- Reduces dependence on synthetic fertilizers, lowering environmental pollution.
3. Boosts Primary Productivity
- Essential for amino acids, proteins, nucleic acids, and chlorophyll.
- Enhances plant growth and biomass production, forming the basis of terrestrial and aquatic food webs.
4. Supports Sustainable Agriculture
- Crop rotation and intercropping with legumes improve nitrogen availability.
- Reduces fertilizer costs and chemical pollution.
5. Provides Ecosystem Services
- Prevents soil erosion by maintaining plant cover.
- Enhances biodiversity by attracting pollinators and supporting herbivores.
- Improves soil structure and moisture retention.
6. Maintains Ecological Balance
- Replenishes nitrogen lost through harvesting, leaching, and denitrification.
- Ensures a continuous nitrogen supply for long-term ecosystem stability.
7. Reduces Environmental Impacts
By minimizing synthetic fertilizer use, nitrogen fixation:
- Decreases greenhouse gas emissions.
- Reduces eutrophication of water bodies.
- Promotes climate resilience.
Factors Influencing Nitrogen Fixation
- Plant species: Legumes like alfalfa and clover fix more nitrogen compared to others.
- Soil conditions: pH, organic matter, and aeration influence microbial activity.
- Climatic factors: Temperature and rainfall affect fixation rates.
- Microbial symbiosis: Efficiency varies depending on rhizobia and host compatibility.
- Residual soil nitrogen: High levels of available nitrogen may suppress fixation.
Conclusion
Nitrogen fixation is the cornerstone of the nitrogen cycle and a foundation of ecosystem productivity. By converting atmospheric N₂ into bioavailable ammonia, microorganisms and nitrogen-fixing plants sustain life on Earth. This natural process enhances soil fertility, supports agriculture, reduces reliance on chemical fertilizers, and helps maintain ecological balance.
In the face of climate change and increasing nitrogen pollution, promoting biological nitrogen fixation through sustainable practices such as legume-based farming, crop rotation, and microbial inoculants is crucial. By harnessing this process, we can protect ecosystems, reduce environmental impacts, and ensure food security for future generations.
Frequently Asked Questions (FAQ)
Q1. What is nitrogen fixation?
Nitrogen fixation is the process of converting inert atmospheric nitrogen gas (N₂) into ammonia (NH₃) or related compounds that plants can use.
Q2. Which organisms fix nitrogen naturally?
Nitrogen fixation is primarily carried out by bacteria (Rhizobium, Azotobacter, Clostridium), archaea, cyanobacteria (Anabaena, Nostoc), and some symbiotic fungi.
Q3. Why is nitrogen fixation important for plants?
Plants cannot use atmospheric nitrogen directly. Fixed nitrogen in the form of ammonium or nitrate is essential for making amino acids, proteins, and chlorophyll, which support plant growth.
Q4. How does nitrogen fixation support agriculture?
It enriches soil naturally, reducing the need for chemical fertilizers, promoting sustainable farming, and lowering environmental impacts.
Q5. Can nitrogen fixation reduce climate change?
Yes, by reducing dependency on synthetic fertilizers, it lowers greenhouse gas emissions and prevents eutrophication, contributing to environmental sustainability.
Also Read
- What is Microbiology? History, Scopes & Applications
- Agriculture Microbiology Research Topics: Innovations Driving Sustainable Farming
- Microbiology Experiments for Students: A Complete Guide
- Branches of Microbiology: An Overview of Key Fields
- Biochar: Properties, Production, and Applications
- Spirulina: The Superfood Microalga with Limitless Potential
- Molecular Blotting: Techniques, Uses, and Significance
- Antibiotics: Introduction, History, Mechanism and Applications
- Food Microbiology Research Topics: Emerging Trends and Future Perspectives
- Latest Research Topics In Microbiology
- Microbiology Notes
- Immunology Quiz
- Bacteriology Notes

