Table of Contents
ToggleIntroduction
Viruses are among the most fascinating yet challenging microorganisms to study. Unlike bacteria, viruses are extremely small, lack independent replication machinery, and remain undetectable during latent or chronic infections. These unique features make virus detection a complex task for researchers, clinicians, and diagnostic laboratories.
Accurate detection of viruses is crucial for early diagnosis, outbreak surveillance, vaccine development, and epidemiological monitoring. Over the years, a wide range of methods have been developed, from traditional microscopy to advanced molecular and nanotechnology-based assays.
This article provides a comprehensive overview of virus detection techniques, their principles, applications, and recent advancements.
Why Are Viruses Harder to Detect Than Bacteria?
Viruses present unique challenges compared to bacteria due to the following reasons:
- Extremely small size – most viruses range from 20–300 nm, making them invisible under light microscopes.
- Dependence on host cells – viruses cannot replicate outside living host cells.
- Detection limits – often only detectable during the acute phase of infection.
- Disappearance before symptoms – some viruses vanish before clinical symptoms appear, complicating diagnosis.
Because of these challenges, virologists use two main approaches:
- Direct detection – Identification of viral particles, proteins, or genetic material.
- Indirect detection – Monitoring immune responses, such as virus-specific antibodies.
Methods of Virus Detection
1. Microscopy
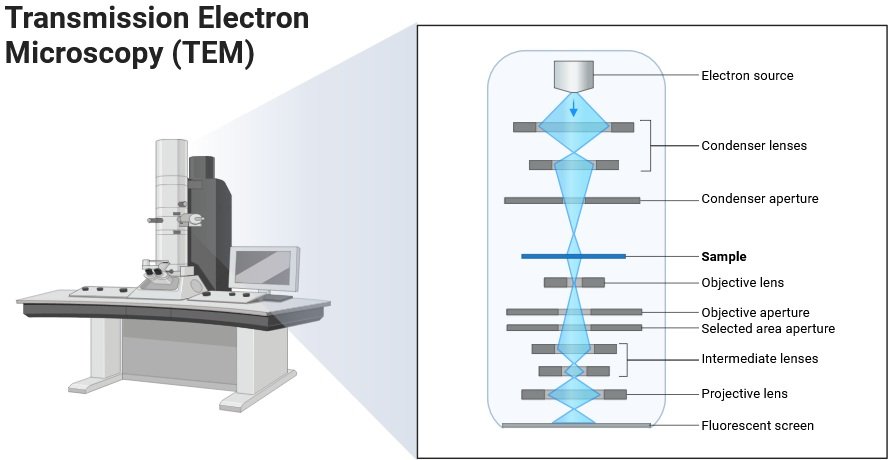
Transmission Electron Microscopy (TEM)
- Provides direct high-resolution images of viral particles.
- Widely used for diagnosis in the 1960s–1990s.
- Currently used for:
- Identifying unknown outbreaks.
- Biopharmaceutical quality control (checking viral contamination).
- Although limited in clinical use today, TEM remains a gold standard for visualizing virus morphology.
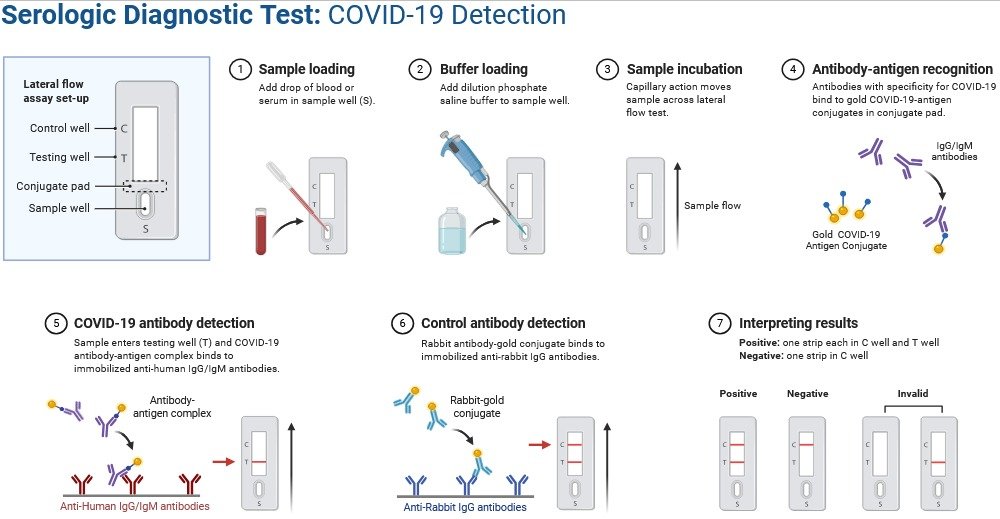
2. Advanced Serological Immunoassays
These assays detect antibodies (IgM, IgG, IgA) or viral antigens using labels such as radioactive, fluorescent, or chemiluminescent tags.
Examples:.
- Hepatitis C Virus (HCV)
- Antibody tests for anti-HCV (indirect).
- Direct detection of core antigen (cAg) or HCV RNA.
- The diagnosis is improved by one step core antigen quantification.
- Dengue Virus (DENV) & Zika Virus (ZIKV)
- Multiplex microsphere immunoassay (MIA) detects viral proteins like NS1 and NS5.
- IgG avidity test distinguishes current Zika infection from past dengue infection.
- Image-based fluorescent neutralization test offers better accuracy than ELISA.
- Hepatitis E Virus (HEV)
- Saliva-based immunoassays (non-invasive alternative to blood testing).
- HEV antigen assays outperform RNA ELISA and anti-HEV IgM assays.
- Respiratory Syncytial Virus (RSV)
- Luciferase Immuno-precipitation System (LIPS) detects antibodies against RSV glycoproteins.
- Useful in tracking infection and vaccine response.
- Flaviviruses (West Nile, Yellow Fever, Dengue, Zika)
- High cross-reactivity complicates detection.
- Microarray-based high-resolution serological tests help differentiate between flavivirus infections.
3. ELISA-Based Immunodetection
Principle: Antigen–antibody interaction tagged with fluorescent, chemiluminescent, or colorimetric signals.
Types of ELISA:
- Standard ELISA.
- Fluorescence Polarization Immunoassay (FPIA).
- Micro Particle Enzyme Immunoassay (MEIA).
- Chemiluminescent Immunoassay (CLIA).
Applications:
- MagLISA: Detects Influenza A virus at ultra-low concentrations using gold nanoparticles with magnetic nanobeads.
- Crimean-Congo Hemorrhagic Fever Virus (CCHFV): Recombinant nucleoprotein-based IgM/IgG ELISA.
- Nipah and Hendra Viruses: Indirect ELISA using recombinant glycoproteins.
- Dengue & Chikungunya: Multiplex colorimetric ELISA for IgM/IgG detection in low-resource settings.
4. Immunofluorescence (IF) Detection
Principle: Fluorescently labelled antibodies bind to viral antigens, which are then seen under UV radiation.
Applications:
- Used for quick virus detection since the 1970s.
- Finds viral antigens and antibodies that are specific to the virus (IgG, IgM, and IgA).
Advancements:
- Recombinant Rabies Virus (rRVGFP): Expresses green fluorescent protein.
- Makes it easier and faster to identify rabies antibodies.
- Compared to the previous RFFIT method, it is simpler and less expensive.
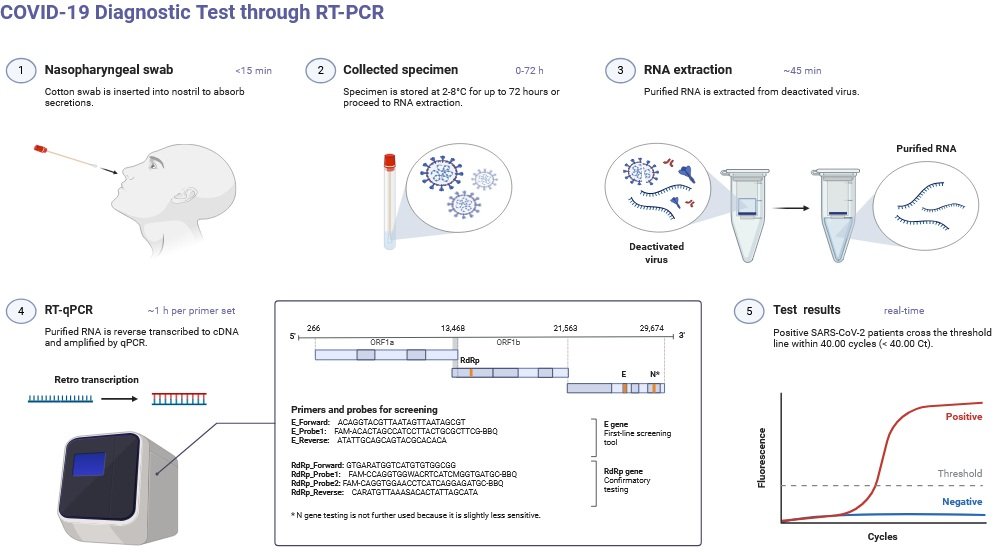
5. PCR and RT-PCR-Based Detection
Principle: Enhances viral nucleic acids (DNA/RNA). Various forms: PCR, RTPCR, qPCR, RTLAMP, RTRPA.
Types: PCR, RT-PCR, qPCR, RT-LAMP, RT-RPA.
Examples & Applications:
- Respiratory Syncytial Virus (RSV): RTRPA (isothermal method) – 96% accuracy compared to qRT-PCR; beneficial in isolated locations.
- Ebola virus: RTRPA with paper microfluidics → multiplex detection of multiple viral RNAs.
- The rabies virus (RABV) is identified by real-time reverse transcription polymerase chain reaction (RTPCR) assays that target the N and L genes. These assays are particularly effective at detecting African isolates.
- Fluorescent RTLAMP with quenching probes for sensitivity equivalent to RTPCR, a quick surveillance tool.
- Trioplex real-time RTPCR for ZIKV/DENV/CHIKV: Trioplex real-time RTPCR simultaneously identifies all three with great sensitivity.
- The RNA in situ hybridization (RNAISH) experiment for the fish virus VHSV is more sensitive than the qRTPCR method.
- Influenza H9N2: One step RT-PCR is a very selective test that does not cross-react with other influenza subtypes.
6. Next-Generation Sequencing (NGS)
Principle: A contemporary sequencing technique that reads entire genomes at a high rate.
Applications in virology include:
- Determines the novel viruses responsible for unexplained illnesses.
- Follows viral epidemics and pandemics, such as influenza and COVID19.
- Aids in comprehending viral transmission and evolution.
- NGS offers more effective monitoring and precise estimations of infection time in HIV1.
- Important takeaway: more accurate and sensitive than earlier genetic techniques.
7. Aptamer-Based Detection and Monoclonal Antibodies (mAbs)
- Monoclonal antibodies: Traditionally used for detecting viral proteins.
- Aptamers: Synthetic DNA/RNA molecules engineered to bind viral proteins with high specificity.
Advantages of aptamers:
- Can be custom-designed for any viral protein.
- More stable and versatile than antibodies.
- Promising tool for detecting viral hepatitis and emerging pathogens.
8. Nanoparticle-Based Detection
Principle: Uses nanomaterials (e.g., gold nanoparticles) for sensitive viral detection.
Applications:
- Lateral Flow Immunoassays (LFIA): Paper-strip tests (similar to pregnancy tests) for rapid point-of-care diagnosis.
- Gold nanoparticle assays: Improve sensitivity, speed, and accessibility of viral diagnostics.
This technology is especially valuable in resource-limited regions.
Conclusion
The detection of viruses is more difficult than that of bacteria because of their minute size, inability to reproduce outside of host cells, and poor detectability during latent or persistent infections. Several strategies have been created throughout the years to address these challenges, including traditional microscopy (TEM), serological immunoassays, and more recent techniques like ELISA, immunofluorescence, PCR/RTPCR, and NGS.
Recent advancements like high-throughput sequencing, aptamers, and nanoparticle-based assays have increased sensitivity, specificity, and the speed of point-of-care diagnostics. Taken together, these methods support vaccine development, outbreak monitoring, epidemiological surveillance, and improved clinical diagnosis.
FAQs on Virus Detection
Q1. Why can’t viruses be detected as easily as bacteria?
Viruses are much smaller, cannot replicate outside host cells, and often remain hidden during latent or chronic stages, making them harder to detect.
Q2. Which method is considered the gold standard for virus detection?
PCR and RT-PCR are widely regarded as gold-standard techniques due to their high sensitivity and specificity.
Q3. How are emerging viruses like COVID-19 detected?
Next-Generation Sequencing (NGS), RT-PCR, and rapid antigen-based assays are primarily used for COVID-19 detection.
Q4. Can serological tests diagnose all viral infections?
No, serological tests detect antibodies, which may take time to appear. They are useful for confirming past infections but less reliable for early detection.
Q5. What are nanoparticle-based tests, and why are they important?
They use nanomaterials like gold nanoparticles for rapid, low-cost detection. These are especially important for point-of-care testing in low-resource settings.
Also Read
- Molecular Blotting: Techniques, Uses, and Significance
- Mycorrhiza: Structure, Types, and Role in Plant Growth
- Rhizobium: The Nitrogen-Fixing Bacterium
- Bacillus subtilis– Morphology, Habitat, Diseases & Industrial Applications
- Basics of Food Microbiology in
- Top 10 Universities in India for Microbiology
- Immune System Cells: The Body’s Cellular Defenders
- Biochar: Properties, Production, and Applications
- Ribonucleic acid (RNA): Types, Structure & their Function
- Antimicrobial Resistance (AMR) Mechanisms and Alternatives
- Spirulina: The Superfood Microalga with Limitless Potential
- Microbiology Notes
- Bacteriology Quiz


Great, can we save this as pdf?
Thank you, Ahmed, for your comment, Yes You can Download Notes as PDF.